Peripheral vascular disease commonly affects the arteries supplying the leg and is mostly caused by atherosclerosis. Restriction of blood flow, due to arterial stenosis or occlusion, often leads patients to complain of muscle pain on walking (intermittent claudication). Any further reduction in blood flow causes ischaemic pain at rest, which affects the foot. Ulceration and gangrene may then supervene and can result in loss of the limb if not treated. The Fontaine score is useful when classifying the severity of ischaemia. Although many patients with claudication remain stable, about 150 200 per million of the population progress to critical limb ischaemia (Fontaine III or IV) each year. Many patients with critical limb ischaemia can undergo revascularisation, which has a reasonable chance of saving the limb. A recent audit by the Vascular Surgical Society found a success rate of over 70% for these patients. However, many patients still require major amputation. Rehabilitation of elderly patients after amputation can prove difficult, with high community costs. Critical limb ischaemia has been estimated to cost over £200m a year in the United Kingdom.
Intermittent claudication
History and examination
A history of muscular, cramp like pain on walking that is rapidly relieved by resting, together with absent pulses, strongly supports the diagnosis of intermittent claudication. Disease of the superficial femoral artery in the thigh results in absent popliteal and foot pulses and often causes calf claudication. Disease of the aorta or iliac artery results in a weak or absent femoral pulse, often associated with a femoral bruit. Disease at this level may cause calf, thigh, or buttock claudication.
The dorsalis pedis artery lies superficially on the dorsum of the foot, although its position varies considerably. The posterior tibial artery lies deeper behind the medial malleolus. Many healthy people have only one foot pulse. The popliteal pulse can be difficult to palpate in muscular patients. A prominent popliteal pulse suggests the possibility of a popliteal aneurysm.
Differential diagnosis
The pain of nerve root compression can be mistaken for vascular claudication. A careful history can usually distinguish nerve root compression, especially sciatica due to compression of the lumbosacral root. However, compression of the cauda equina due to spinal stenosis can be more difficult to diagnose. This condition usually causes pain that radiates down both legs. Although the pain is made worse by walking, it also comes on after prolonged standing and is not rapidly relieved by rest, unlike vascular claudication.
Investigation
There are many causes of leg pain that can occur in the presence of asymptomatic peripheral vascular disease.
Therefore, the absence of pulses does not necessarily imply a causal link. Furthermore, the presence of pulses at rest does not exclude symptomatic peripheral vascular disease. A good history together with an ankle brachial systolic pressure index of less than 0.9 confirms the diagnosis. Exercise testing provides an objective measurement of walking distance, and highlights other exercise limiting conditions such as arthritis and breathlessness. However, exercise testing takes time, and many patients find it difficult or impossible to walk on a treadmill. Only those with a good history of claudication and normal resting ankle brachial
systolic pressure indexes require an exercise test. Duplex ultrasound scanning is useful for delineating the anatomical site of disease in the lower limb. Many hospitals still use arteriography for this purpose or when the results of duplex scanning are equivocal. This invasive and expensive investigation should not be requested unless there is a plan to proceed with revascularisation, if possible.
Principles of treatment
Intermittent claudication seems a relatively benign condition, although severe claudication may preclude patients from manual work. The risk of generalised vascular disease is probably more important. Patients with claudication have a three times higher risk of death compared with age matched controls.Modification of risk factors is therefore vital to reduce death from myocardial infarction and stroke. All patients should be advised to stop smoking and take regular exercise. They should also be screened for hyperlipidaemia and diabetes. Patients with peripheral vascular disease benefit from regular chiropody, and those with diabetes should attend a foot clinic.Obesity reduces exercise capacity, and losing weight will improve the walking distance.
Exercise programmes
A recent meta analysis of 21 supervised exercise programmes showed that training for at least six months, by walking to near maximum pain tolerance, significantly improved pain free and maximum walking distances. The only controlled trial comparing an exercise programme with percutaneous transluminal angioplasty found that exercise was better. Exercise programmes are cheaper than percutaneous transluminal angioplasty or surgery, although long term compliance seems poor.
Endovascular techniques
The number of percutaneous transluminal angioplasties performed for claudication has risen steeply in recent years. In some situations endovascular techniques have virtually replaced conventional surgery. Percutaneous transluminal angioplasty seems best suited for stenoses or short occlusions of the iliac and superficial femoral vessels, with one year patency rates of 90% and 80% respectively. Angioplasty carries a small but definite risk of losing the limb because of thrombosis or embolisation, and patients should be informed of this risk.
Metallic stents push back the atheroma and improve on the initial lumen gain after angioplasty alone. The indications for iliac stents include a residual stenosis or dissection after angioplasty and long occlusions, but there seems little evidence to justify their routine use. Deployment of stents more distally has produced disappointing results due to high restenosis rates.
Surgery
The role of bypass for longer arterial occlusions remains poorly defined because of a lack of proper trials comparing it with percutaneous transluminal angioplasty and conservative treatment. Polyester (Dacron) aortobifemoral bypass grafts have five year patency rates of over 90% but are associated with a mortality of up to 5%. Complications include graft infection and postoperative impotence. Femoropopliteal bypass grafting, using autologous long saphenous vein, polyester, or polytetrafluoroethylene (Goretex) yields patency rates of less than 70% at five years. The early patency of prosthetic grafts seems similar to that of vein grafts, although the long term results seem less good. Femoropopliteal bypass grafts should rarely be used for patients with claudication.
Critical limb ischaemia
History and examination
Patients with critical limb ischaemia often describe a history of deteriorating claudication, progressing to nocturnal rest pain. Ulceration or gangrene commonly results from minor trauma. Nocturnal rest pain often occurs just after the patient has fallen asleep when the systemic blood pressure falls, further reducing perfusion to the foot. Hanging the foot out of bed increases perfusion and produces the typical dusky red hue due to loss of capillary tone. Elevation causes pallor and venous guttering. Inspect the foot carefully for ulceration under the heel andbetween the toes. Swelling suggests deep infection. If you can palpate foot pulses consider an alternative cause of pain, such as gout. Patients with critical limb ischaemia require urgent referral to a vascular surgeon.
Investigation
The ankle brachial systolic pressure index is usually less than 0.5. Arterial calcification may result in falsely increased pressures, and caution is needed when relying on Doppler pressures alone, especially in diabetic patients. All patients with critical limb ischaemia should ideally have arteriography with a view to endovascular treatment, if feasible. Duplex scanning may be used instead of angiography and for mapping of the long saphenous vein before distal bypass surgery. Dependent Doppler or pulse generated run off can help to determine the most suitable artery to receive a distal bypass graft if these cannot be identified by angiography.
Principles of treatment
The same principles and techniques used to treat claudication also apply to critical limb ischaemia. However, critical limb ischaemia is usually caused by multilevel disease, which means that success rates are lower. Treatment focuses on saving the limb, although modification of risk factors remains important.
Endovascular treatment
Percutaneous transluminal angioplasty or stenting of proximal disease may relieve ischaemic rest pain, but healing of ulceration or gangrene usually requires restoration of foot pulses. This may necessitate extensive angioplasty of the superficial femoral, popliteal, and tibial arteries. Good results have been reported with subintimal angioplasty. Endovascular treatment can also reduce the magnitude of subsequent surgery.
Surgery
Patients with a pattern of arterial disease considered unsuitable for endovascular treatment will usually require surgery. Fit patients with proximal disease benefit greatly from aortobifemoral bypass grafting. In unfit patients the options include crossfemoral bypass for unilateral disease or axillobifemoral bypass for bilateral disease. These extra anatomic procedures have lower patency rates. Many patients with distal disease will require bypass grafting to the popliteal or crural arteries below the knee. Autologous vein grafts give the best patency rates (70% at one year). Postoperative duplex surveillance may improve patency by permitting the detection and treatment of vein graft stenoses before occlusion occurs.
Amputation
Patients with unreconstructable peripheral vascular disease, fixed flexion deformities, or extensive tissue loss usually require a major amputation. Preservation of the knee joint has enormous advantages for wearing artificial limbs and subsequent mobility. However, there is little point in risking a non healing, below knee amputation if the patient seems unlikely to walk again. Similarly, a patient with good prospects of wearing an artificial limb will fare better with an above knee amputation, if below knee amputation seems unachievable. Local amputation of ulcerated or gangrenous toes will not heal without revascularisation.
Pain relief
Critical limb ischaemia causes severe pain that requires narcotic analgesia to provide relief. A slow release opiate such as morphine seems a good option. Opiates can be supplemented by non steroidal anti
inflammatory drugs if these are not contraindicated. Apart from rehydration, adequate analgesia alone may be the best treatment for patients with dementia or other severe comorbidity. If opiate analgesia remains inadequate, then lumbar sympathectomy (surgical or chemical) or spinal cord stimulation may help. About 20
30% of patients with critical limb ischaemia have unreconstructable disease. A meta analysis of six randomised trials of Iloprost, a stable prostacyclin analogue, found that infusion of this drug reduced the death and amputation rate. Phantom limb pain may complicate major amputation. Amitryptyline, carbamazepine, transcutaneous nerve stimulation, and acupuncture can help in this situation.
<a href="http://onlinemallstore.blogspot.com/2011/06/perfume.html/%22%3Eperfume%3C/a>
Friday, October 29, 2010
Thursday, October 28, 2010
ABC of arterial and venous disease Acute limb ischaemia
Limb ischaemia is classified on the basis of onset and severity. Complete acute ischaemia will lead to extensive tissue necrosis within six hours unless the limb is surgically revascularised. Incomplete acute ischaemia can usually be treated medically in the first instance. Patients with irreversible ischaemia require urgent amputation unless it is too extensive or the patient too ill to survive.
Clinical features
Apart from paralysis (inability to wiggle toes or fingers) and anaesthesia (loss of light touch over the dorsum of the foot or hand), the symptoms and signs of acute ischaemia are non specific or inconsistently related to its completeness. Pain on squeezing the calf indicates muscle infarction and impending irreversible ischaemia. Acute arterial occlusion is associated with intense spasm in the distal arterial tree, and initially the limb will appear “marble” white. Over the next few hours, the spasm relaxes and the skin fills with deoxygenated blood leading to mottling that is light blue or purple, has a fine reticular pattern, and blanches on pressure. At this stage the limb is still salvageable. However, as ischaemia progresses, stagnant blood coagulates leading to mottling that is darker in colour, coarser in pattern, and does not blanch. Finally, large patches of fixed staining progress to blistering and liquefaction. Attempts to revascularise such a limb are futile and will lead to life threatening reperfusion injury. In cases of real doubt the muscle can be examined at surgery through a small fasciotomy incision. It is usually obvious when the muscle is dead.
Aetiology
Acute limb ischaemia is most commonly caused by acute thrombotic occlusion of a pre existing stenotic arterial segment (60% of cases) or by embolus (30%). Distinguishing these two conditions is important because treatment and prognosis are different. Other causes are trauma, iatrogenic injury, popliteal aneurysm, and aortic dissection. More than 80% of peripheral emboli arise from the left atrial appendage in association with atrial fibrillation. They may also arise from the left ventricle, heart valves, prosthetic bypass grafts, aneurysmal disease, paradoxical embolism, and atrial myxoma (rare). In 15% of cases the source of embolus is obscure. Thrombosis in situ may arise from acute plaque rupture, hypovolaemia, or pump failure (see below).
Management
General measures
When a patient is suspected to have an acutely ischaemic limb the case must be discussed immediately with a vascular surgeon. A few hours can make the difference between death or amputation and complete recovery of limb function. If there are no contraindications (acute aortic dissection or multiple trauma, particularly serious head injury) give an intravenous bolus of heparin to limit propagation of thrombus and protect the collateral circulation.
Is angiography required?
If ischaemia is complete, the patient must be taken directly to the operating theatre because angiography will introduce delay, thrombolysis is not an option, and lack of collateral flow will prevent visualisation of the distal vasculature. If ischaemia is incomplete the patient should have preoperative angiography
since simple embolectomy or thrombectomy is unlikely to be successful, thrombolysis may be an option, and the surgeon requires a “road map” for distal bypass.
Acute embolus
Embolic occlusion of the brachial artery is not usually limb threatening, and in elderly people non
operative treatment is reasonable. Younger patients should have embolectomy to prevent subsequent claudication, especially if the dominant arm is affected. A leg affected by embolus is nearly always threatened and requires immediate surgical revascularisation. Emboli usually lodge at the common femoral bifurcation or, less commonly, the popliteal trifurcation. Femoral embolus is associated with profound ischaemia to the level of the upper thigh because the deep femoral artery is also affected. A femoral pulse does not exclude the diagnosis. Embolectomy can be done under local, regional, or general anaesthetic. The adequacy of embolectomy should be confirmed by angiography while the patient is on the operating table. On table thrombolysis should be considered if mechanical clearance has been unsuccessful. If the embolus has occurred in an area of longstanding atherosclerotic disease, surgical bypass may be necessary. Postoperatively the patient should continue to receive heparin to prevent formation of further emboli. Many surgeons postpone heparin for six hours after surgery to reduce the risk of a haematoma forming.Warfarin reduces the risk of recurrent embolism, and unless contraindicated, should be prescribed to all patients long term. Patients should not be given warfarin without first being on heparin for 48 hours since warfarin can produce a transient procoagulant state due to inhibition of the vitamin K dependent anticoagulant proteins C and S. Opinions differ about how thorough you should be in establishing the source of emboli. Transthoracic echocardiography is poor at detecting a thrombus in patients with atrial fibrillation, and a negative result does not exclude the diagnosis. Transoesophageal echocardiography provides excellent views of the left atrium but is moderately invasive and not universally available. In patients with suspected paroxysmal tachyarrhythmias, 24 hour electrocardiographic monitoring should be considered. Even if no source of embolism is found,
anticoagulation should continue long term. Although immediate loss of a limb after correctly managed acute embolus is unusual, many series report a 10 20% in hospital mortality from heart failure or recurrent embolism, particularly stroke
.
Saddle embolus
Patients with acute embolic occlusion of the aortic bifurcation have femoral pulses and appear marble white or mottled to the waist. They may also present with paraplegia due to ischaemia of the cauda equina, which can be irreversible. Immediate bilateral embolectomy restores lower limb perfusion, but many patients subsequently die from reperfusion injury.
Popliteal aneurysm
A popliteal aneurysm can initiate acute ischaemia by forming a thrombus or acting as a source of emboli. Thrombolysis is often the best treatment as simple embolectomy or thrombectomy usually leads to early rethrombosis and surgical bypass is often precluded by obliteration of the distal run off. Once the circulation is restored, a bypass should be performed to exclude the aneurysm.
Atheroembolism
Cholesterol emboli are shed from a complex, often acutely ruptured, atherosclerotic plaque. Distal pulses are usually present. The patient characteristically presents with the blue toe (finger) syndrome, which may mimic Raynaud’s phenomenon. If the blue toe syndrome is not recognised patients may deteriorate rapidly and require amputation.
Thrombosis in situ
Limbs affected by stable chronic ischaemia do not usually suddenly deteriorate without a reason—for example, silent myocardial infarction or underlying, hitherto asymptomatic, malignancy. Septicaemia, particularly pneumococcal and meningococcal, may be associated with widespread thrombosis.
Trauma
The commonest causes of non iatrogenic injury are limb fractures and dislocations (supracondylar fractures of the humerus in children, tibial fractures in adults), blunt injuries occurring in road traffic accidents, and stab wounds. In the United Kingdom, acute traumatic limb ischaemia is often iatrogenic, being caused by arterial cannulation (coronary angioplasty, aortic balloon pump), vascular and orthopaedic procedures on the limb (especially if exsanguinating tourniquets are used), or pelvic surgery (cystectomy, anterior resection) in patients with subclinical aortoiliac disease in whom the ligated pelvic collaterals form the main blood supply to the legs. Postoperative assessment of lower limb ischaemia may be confused by the presence of epidural or spinal anaesthesia. The presence of distal pulses does not exclude serious arterial injury. Pulse oximetry, Doppler signals, and measurement of the ankle brachial pressure index may be helpful, but in cases of doubt, proceed to angiography.
Intra-arterial drug administration
Intra-arterial drug administration leads to intense spasm and microvascular thrombosis. The leg is mottled and digital gangrene is common, but pedal pulses are usually palpable. The mainstay of treatment is supportive care, hydration to minimise renal failure secondary to rhabdomyolysis, and full heparinisation. Vascular reconstruction is almost never indicated, but fasciotomy may be required to prevent a compartment syndrome.
Venous gangrene
Venous gangrene can be mistaken for acute limb ischaemia. However, the leg is invariably swollen and the superficial veins full. Oedema may make it impossible to palpate pedal pulses, but Doppler examination will show normal distal waveforms and pressures. Management includes elevation, heparinisation, thrombolysis, and treatment of the underlying cause (usually pelvic or abdominal malignancy).
Aortic dissection
This may cause upper and lower limb ischaemia due to pinching of the ostia of the relevant arteries by the false lumen.
Thoracic outlet syndrome
Pressure on the subclavian artery from a cervical rib or abnormal soft tissue band may lead to a post
stenotic dilatation lined with thrombus, which predisposes to occlusion or embolisation. The distal circulation may be chronically obliterated and digital ischaemia advanced before the thoracic outlet syndrome is diagnosed. The diagnosis is based on the results of duplex ultrasonography or angiography, or both. Treatment options include thrombolysis, thrombectomy or embolectomy, excision of the cervical rib, and repair of the aneurysmal segment.
Thrombolysis
In thrombolysis a cannula is embedded into the distal extent of the thrombus and streptokinase or, preferably, recombinant tissue plasminogen activator is infused. The technique cannot be used in patients with complete ischaemia because thrombus dissolution takes several hours. It is also relatively ineffective against the organised thrombus present in most peripheral emboli and is associated with an appreciable minor (20%,
mainly groin haematoma) and major (5%, serious haemorrhage and stroke) complication rate. Thrombolysis should be undertaken only in an environment where experienced nursing and medical staff can closely monitor the patient.
Investigations of venous disease
Venous thrombosis
Colour Duplex scanning is both sensitive and specific (90 100% in most series) for detecting proximal deep vein thrombosis. Deep veins and arteries lie together in the leg, and the normal vein appears as an echo free channel and is usually larger than the accompanying artery. Venous ultrasonography is a very accurate method ofidentifying deep vein thrombi from the level of the common femoral vein at the groin crease to the popliteal vein but is less reliable for diagnosing calf vein thrombosis.
Criteria for diagnosis of deep vein thrombosis
x Failure of vein to collapse on direct compression
x Visualisation of thrombus within lumen
x Absent or abnormal venous pulsation on Doppler scanning
Venous reflux
Colour duplex scanning has revolutionised the investigation of the lower limb venous system because it allows instant visualisation of blood flow and its direction. Thus, reflux at the saphenofemoral junction, saphenopopliteal junction, and within the deep venous system, including the popliteal vein beneath the knee and the gastrocnemius veins, can be detected without invasive techniques. Although venous reflux can be assessed with a pencil Doppler, this technique misses 12% of saphenofemoral and 20% of saphenopopliteal junction reflux compared with colour duplex scanning.
Colour Duplex scanning is both sensitive and specific (90 100% in most series) for detecting proximal deep vein thrombosis. Deep veins and arteries lie together in the leg, and the normal vein appears as an echo free channel and is usually larger than the accompanying artery. Venous ultrasonography is a very accurate method ofidentifying deep vein thrombi from the level of the common femoral vein at the groin crease to the popliteal vein but is less reliable for diagnosing calf vein thrombosis.
Criteria for diagnosis of deep vein thrombosis
x Failure of vein to collapse on direct compression
x Visualisation of thrombus within lumen
x Absent or abnormal venous pulsation on Doppler scanning
Venous reflux
Colour duplex scanning has revolutionised the investigation of the lower limb venous system because it allows instant visualisation of blood flow and its direction. Thus, reflux at the saphenofemoral junction, saphenopopliteal junction, and within the deep venous system, including the popliteal vein beneath the knee and the gastrocnemius veins, can be detected without invasive techniques. Although venous reflux can be assessed with a pencil Doppler, this technique misses 12% of saphenofemoral and 20% of saphenopopliteal junction reflux compared with colour duplex scanning.
ABC OF ANTENATAL CARE
Organisation of antenatal care
Looking after pregnant women presents one of the paradoxes of modern medicine. Normal women proceeding through an uneventful pregnancy require little formal medicine. Conversely, those at high risk of damage to their own health or that of their fetus require the use of appropriate scientific technology. Accordingly, there are two classes of women, the larger group requiring support but not much intervention and the other needing the full range of diagnostic and therapeutic measures as in any other branch of medicine. To distinguish between the two is the aim of a well run antenatal service. Antenatal clinics provide a multiphasic screening service; the earlier women are screened to identify those at high risk of specified problems the sooner appropriate diagnostic tests can be used to assess such women and their fetuses and treatment can be started. As always in medicine, diagnosis must precede treatment, for unless the women who require treatment can be identified specifically, management cannot be correctly applied.
Background
Some women attend for antenatal care because it is expected of them. They have been brought up to believe that antenatal care is the best way of looking after themselves and their unborn children. This is reinforced in all educational sources from medical textbooks to women’s magazines. Prenatal care started in Edinburgh at the turn of the 20th century, but clinics for the checking of apparently well pregnant women were rare before the first world war. During the 1920s a few midwifery departments of hospitals and interested general practitioners saw women at intervals to check their urine for protein. Some palpated the abdomen, but most pregnant women had only a medical or midwifery consultation once before labour, when they booked. Otherwise, doctors were concerned with antenatal care only “if any of the complications of pregnancy should be noticed”. Obstetrics and midwifery were first aid services concerned with labour and its complications: virtually all vigilance, thought, and attention centred on delivery and its mechanical enhancement. Little attention was paid to the antenatal months. During the 1920s a wider recognition emerged of the maternal problems of pregnancy as well as those of labour; the medical profession and the then Ministry of Health woke up to realise that events of labour had their precursors in pregnancy. Janet Campbell, one of the most farsighted and clear thinking women in medicine, started a national system of antenatal
clinics with a uniform pattern of visits and procedures; her pattern of management can still be recognised today in all the clinics of the Western world. Campbell’s ideas became the clinical obstetric screening service of the 1930s. To it has been added a series of tests, often with more enthusiasm than scientific justification; over the years few investigations have been taken away, merely more added. Catalysed by the National Perinatal Epidemiological Unit in Oxford, various groups of more thoughtful obstetricians have tried to sort out which of the tests are in fact useful in predicting fetal and maternal hazards and which have a low return for effort. When this has been done a rational antenatal service may be developed, but until then we must work with a confused service that “growed like Topsy”. It is a mixture of the traditional clinical laying on of hands and a
Some women attend for antenatal care because it is expected of them. They have been brought up to believe that antenatal care is the best way of looking after themselves and their unborn children. This is reinforced in all educational sources from medical textbooks to women’s magazines. Prenatal care started in Edinburgh at the turn of the 20th century, but clinics for the checking of apparently well pregnant women were rare before the first world war. During the 1920s a few midwifery departments of hospitals and interested general practitioners saw women at intervals to check their urine for protein. Some palpated the abdomen, but most pregnant women had only a medical or midwifery consultation once before labour, when they booked. Otherwise, doctors were concerned with antenatal care only “if any of the complications of pregnancy should be noticed”. Obstetrics and midwifery were first aid services concerned with labour and its complications: virtually all vigilance, thought, and attention centred on delivery and its mechanical enhancement. Little attention was paid to the antenatal months. During the 1920s a wider recognition emerged of the maternal problems of pregnancy as well as those of labour; the medical profession and the then Ministry of Health woke up to realise that events of labour had their precursors in pregnancy. Janet Campbell, one of the most farsighted and clear thinking women in medicine, started a national system of antenatal
clinics with a uniform pattern of visits and procedures; her pattern of management can still be recognised today in all the clinics of the Western world. Campbell’s ideas became the clinical obstetric screening service of the 1930s. To it has been added a series of tests, often with more enthusiasm than scientific justification; over the years few investigations have been taken away, merely more added. Catalysed by the National Perinatal Epidemiological Unit in Oxford, various groups of more thoughtful obstetricians have tried to sort out which of the tests are in fact useful in predicting fetal and maternal hazards and which have a low return for effort. When this has been done a rational antenatal service may be developed, but until then we must work with a confused service that “growed like Topsy”. It is a mixture of the traditional clinical laying on of hands and a
patchily applied provision of complex tests, whose availability often depends as much on the whims of a health authority’s ideas of financial priority as on the needs of the women and their fetuses. As well as these economic considerations, doctors planning the care of women in pregnancy should consider the women’s own wishes. Too often antenatal clinics in the past have been designated cattle markets; the wishes of women coming for care should be sought and paid attention to. A recurrent problem is the apparent rush of the hospital clinic. The waiting time is a source of harassment and so is the time taken to travel to the clinic. Most women want time and a rapport with the antenatal doctor or midwife to ask questions and have them answered in a fashion they can understand. It is here that the midwives
come into their own for they are excellent at the care of women undergoing normal pregnancies. In many parts of the country midwives run their own clinics in places where women would go as part of daily life. Here,
midwives see a group of healthy normal women through pregnancy with one visit only to the hospital antenatal clinic.To get the best results, women at higher risk need to be screened out at or soon after booking. They will receive intensive care at the hospital consultant’s clinic and those at intermediate risk have shared care between the general practitioner and the hospital. The women at lower risk are seen by the midwives at the community clinics. Programmes of this nature now run but depend on laying down protocols for care agreed by all the obstetricians, general practitioners and midwives. Co-operation and agreement between the three groups of carers, with mutual respect and acceptance of each other’s roles, are essential. Janet Campbell started something in 1920. We should not necessarily think that the pattern she derived is fixed forever, and in the new century we may start to get it right for the current generation of women.
come into their own for they are excellent at the care of women undergoing normal pregnancies. In many parts of the country midwives run their own clinics in places where women would go as part of daily life. Here,
midwives see a group of healthy normal women through pregnancy with one visit only to the hospital antenatal clinic.To get the best results, women at higher risk need to be screened out at or soon after booking. They will receive intensive care at the hospital consultant’s clinic and those at intermediate risk have shared care between the general practitioner and the hospital. The women at lower risk are seen by the midwives at the community clinics. Programmes of this nature now run but depend on laying down protocols for care agreed by all the obstetricians, general practitioners and midwives. Co-operation and agreement between the three groups of carers, with mutual respect and acceptance of each other’s roles, are essential. Janet Campbell started something in 1920. We should not necessarily think that the pattern she derived is fixed forever, and in the new century we may start to get it right for the current generation of women.
Styles of antenatal care
The type of antenatal care that a woman and her general practitioner plan will vary with local arrangements. The important first decision on which antenatal care depends is where the baby will be delivered. Ninety seven per cent of babies in the UK are now delivered in institutions, a third of the 2.2% of domiciliary deliveries are unplanned, so about 1.5% are booked as home deliveries. If the delivery is to be in an institution there is still the choice in some areas of general practitioner deliveries either at a separate unit run by general
practitioners isolated from the hospital or in a combined unit with a consultant. Most deliveries take place in an NHS hospital under the care of a consultant team. A small but possibly increasing number in the next few years may be delivered in private care, by a general practitioner obstetrician, a consultant obstetrician, or an independent midwife. Recently a series of midwife led delivery units have been established with no residential medical cover. Once the plans for delivery are decided, the pattern of antenatal visits can be worked out. If general practitioners or midwives are going to look after delivery, antenatal care might be entirely in their hands, with the use of the local obstetric unit for investigations and consultation. At the other end of the
spectrum, antenatal care is in the hands of the hospital unit under a consultant obstetrician and a team of doctors and midwives, the general practitioner seeing little of the woman until she has been discharged from hospital after delivery. Most women, however, elect for antenatal care between these two extremes. They often wish to take a bigger part in their own care. In some antenatal clinics the dipstick test for proteinuria is done by the woman herself. As well as providing some satisfaction, this reduces the load and waiting time at the formal antenatal visit. During pregnancy there may be visits, at certain agreed stages of gestation, to the hospital antenatal clinic for crucial checks, and for the rest of the time antenatal care is performed in the general practitioner’s surgery or midwives’ clinic. These patterns of care keep the practitioner involved in the obstetric care of the woman and allow the woman to be seen in slightly more familiar surroundings and more swiftly. In some areas clinics outside the hospital are run by community midwives; these are becoming increasingly popular. Home antenatal care visits also take place, including the initial booking visit. Delivery may be in the hospital by the consultant led team, by a general practitioner obstetrician, or by a midwife. It is wise, with the introduction of Crown indemnity, that all general practitioner obstetricians have honorary contracts with the hospital obstetric department that they attend to supervise or perform deliveries. About 2% of women now have a home delivery. More than half of these are planned and for this group, antenatal care may well be midwifery led (see ABC of Labour Care).
spectrum, antenatal care is in the hands of the hospital unit under a consultant obstetrician and a team of doctors and midwives, the general practitioner seeing little of the woman until she has been discharged from hospital after delivery. Most women, however, elect for antenatal care between these two extremes. They often wish to take a bigger part in their own care. In some antenatal clinics the dipstick test for proteinuria is done by the woman herself. As well as providing some satisfaction, this reduces the load and waiting time at the formal antenatal visit. During pregnancy there may be visits, at certain agreed stages of gestation, to the hospital antenatal clinic for crucial checks, and for the rest of the time antenatal care is performed in the general practitioner’s surgery or midwives’ clinic. These patterns of care keep the practitioner involved in the obstetric care of the woman and allow the woman to be seen in slightly more familiar surroundings and more swiftly. In some areas clinics outside the hospital are run by community midwives; these are becoming increasingly popular. Home antenatal care visits also take place, including the initial booking visit. Delivery may be in the hospital by the consultant led team, by a general practitioner obstetrician, or by a midwife. It is wise, with the introduction of Crown indemnity, that all general practitioner obstetricians have honorary contracts with the hospital obstetric department that they attend to supervise or perform deliveries. About 2% of women now have a home delivery. More than half of these are planned and for this group, antenatal care may well be midwifery led (see ABC of Labour Care).
Early diagnosis of pregnancy
When a woman attends a practitioner thinking that she is pregnant, the most common symptoms are not always amenorrhoea followed by nausea. Many women, particularly the multiparous, have a subtle sensation that they are pregnant a lot earlier than the arrival of the more formal symptoms and signs laid down in textbooks. Traditionally, the doctor may elicit clinical features, but most now turn to a pregnancy test at the first hint of pregnancy. Symptoms The symptoms of early pregnancy are nausea, increased sensitivity of the breasts and nipples, increased frequency of micturition, and amenorrhoea.
Signs
The doctor may notice on examination a fullness of the breasts with early changes in pigmentation and Montgomery’s tubercuiles in the areola. The uterus will not be felt through the abdominal wall until about 12 weeks of pregnancy. On bimanual assessment uterine enlargement is detectable before this time while cervical softening and a cystic, generally soft feeling of the uterus can be detected by eight weeks. This more subtle sign is not often sought as vaginal examination is not usually performed on a normal woman at this time.
Tests
Mostly the diagnosis of pregnancy is confirmed by tests checking for the higher concentrations of human chorionic gonadotrophin that occur in every pregnancy. The old biological tests using rabbits and frogs are now gone and have been replaced by immunological tests. These depend on the presence of human chorionic gonadotrophin in the body fluids, which is reflected in the urine. The more sensitive the test, the more likely it is to pick up the hormone at lower concentrations—that is, earlier in pregnancy. Enzyme linked immunosorbent assay (ELISA) is the basis of many of the commercial kits currently available in chemist
shops. The assay depends on the double reaction of standard phase antibody with enzyme labelled antibody, which is sensitive enough to detect very low concentrations of human chorionic gonadotrophin. Positive results may be therefore detectable as early as 10 days after fertilisation—that is, four days before the first missed period. Vaginal ultrasound can detect a sac from five weeks and a fetal cardiac echo a week or so later (Chapter 4), but this would not be used as a screening pregnancy test.
Conclusion
At the end of the preliminary consultation women may ask questions about the pregnancy and the practitioner will deal with these. Most of these queries will be considered in the chapter on normal antenatal management. For most women the onset of pregnancy is a desired and happy event, but for a few it may not be so and practitioners, having established a diagnosis, may find that they are then asked to advise on termination of pregnancy. This they should do if their views on the subject allow; if not, they should arrange for one of their partners to discuss it with the patient. Most women, however, will be happy to be pregnant and looking forward to a successful outcome.
Investigations of arterial disease
Ankle brachial pressure index
Under normal conditions, systolic blood pressure in the legs is equal to or slightly greater than the systolic pressure in the upper limbs. In the presence of an arterial stenosis, a reduction in pressure occurs distal to the lesion. The ankle brachial pressure index, which is calculated from the ratio of ankle to brachial systolic pressure, is a sensitive marker of arterial insufficiency. The highest pressure measured in any ankle artery is used as the numerator in the calculation of the index; a value >1.0 is normal and a value < 0.9 is abnormal. Patients with claudication tend to have ankle brachial pressure indexes in the range 0.5 0.9, whereas those with critical ischaemia usually have an index of < 0.5. The index also has prognostic significance because of the association with arterial disease elsewhere, especially coronary heart disease.
Diabetic limbs
Systolic blood pressure in the lower limbs cannot be measured reliably when the vessels are calcified and incompressible—for example, in patients with diabetes—as this can result in falsely high ankle pressures. An alternative approach is to use either the pole test or measurement of toe pressures. Normal toe systolic pressure ranges from 90 100 mm Hg and is 80 90% of brachial systolic pressure. A toe systolic pressure < 30 mm Hg indicates critical ischaemia.
Walk test
Exercise testing will assess the functional limitations of arterial stenoses and differentiate occlusive arterial disease from other causes of exercise induced lower limb symptoms—for example, neurogenic claudication secondary to spinal stenosis. A limited inflow of blood in a limb with occlusive arterial disease results
in a fall in ankle systolic blood pressure during exercise induced peripheral vasodilatation. The walk test is performed by exercising the patient for 5 minutes, ideally on a treadmill, but walking the patient in the
surgery or marking time on the spot are adequate. The ankle brachial pressure index is measured before and after exercise. A pressure drop of 20% or more indicates significant arterial disease. If there is no drop in ankle systolic pressure after a 5 minute brisk walk, the patient does not have occlusive arterial disease proximal to the ankle in that limb.
Duplex scanning
Duplex ultrasonography has a sensitivity of 80% and a specificity of 90 100% for detecting femoral and popliteal disease compared with angiography, but it is less reliable for assessing the severity of stenoses in the tibial and peroneal arteries. Duplex scanning is especially useful for assessing the carotid arteries and for surveillance of infrainguinal bypass grafts where sites of stenosis can be identified before complete graft occlusion occurs and before there is a fall in ankle brachial pressure index. The normal velocity within a graft conduit is 50 120 cm/s. As with native arteries, a twofold increase in peak systolic velocity indicates a stenosis of 50% or more. A peak velocity < 45 cm/s occurs in grafts at high risk of failure.
Identification of distal vessels for arterial bypass grafting
In critically ischaemic limbs, where occlusive disease tends to be present at multiple levels, arteriography often fails to show patent calf or pedal vessels as potential outflows for femorodistal bypass grafting. Alternative non invasive approaches have been developed for preoperative assessment, including pulse generated run off and dependent Doppler assessment.
Transcranial Doppler ultrasonography
Lower frequency Doppler probes (1 2 MHz) can be used to obtain information about blood flow in arteries comprising the circle of Willis and its principal branches. Mean flow velocities > 80 cm/s in the middle cerebral artery, or > 70 cm/s in the posterior and basilar arteries, indicate a serious stenosis. Transcranial Doppler scanning has several applications but is especially useful for intraoperative and postoperative monitoring of patients having carotid endarterectomy.
Helical or spiral computed tomography
Spiral computed tomography is a new, minimally invasive technique for vascular imaging that is made possible by combining two recent advances: slip ring computed tomography (which allows the x ray tube detector apparatus to rotate continuously) and computerised three dimensional reconstruction. A helical scan can cover the entire region of interest (for example, the abdominal aorta from the diaphragm to the iliac bifurcation) in one 30 40 second exposure, usually in a single breath hold. This minimises motion artefact and allows all the scan data to be collected during the first pass of an intravenous bolus of contrast through the arterial tree—that is during the time of maximal arterial opacification. A large number of finely spaced slices from one scan can then be reconstructed to produce high quality two or three dimensional images of the contrast enhanced vessels.
Magnetic resonance angiography
Magnetic resonance angiography has developed rapidly over the past five years. It has the advantage of imaging a moving column of blood and does not require ionising radiation or iodinated contrast, but the technique has obvious drawbacks in terms of cost efficiency and accessibility to scanners. A variety of
imaging sequences are used depending on the vessels being studied and the field strength of the machine. The most commonly used techniques include time of flight, two and three dimensional angiography and phase contrast. Use of a magnetic resonance imaging scanner with a high field strength (which allows rapid acquisition of data) and a carefully timed bolus of gadolinium contrast enables high quality angiographic images to be obtained in a single breath hold. Magnetic resonance angiography is well established for examining the cerebral vessels and the car
ABC of arterial and venous disease
Noninvasive methods of arterial and venous assessment
Although diagnostic and therapeutic decisions in patients with vascular disease are guided primarily by the history and physical examination, the use of non invasive investigations has increased significantly in recent years, mainly as a result of technological advances in ultrasonography. This article describes the main investigative techniques.
Principles of vascular ultrasonography
In the simplest form of ultrasonography, ultrasound is transmitted as a continuous beam from a probe that contains two piezoelectric crystals. The transmitting crystal produces ultrasound at a fixed frequency (set by the operator according to the depth of the vessel being examined), and the receiving crystal vibrates in response to reflected waves and produces an output voltage. Conventional B mode (brightness mode) ultrasonography records the ultrasound waves reflected from tissue interfaces, and a two dimensional picture is built up according to the reflective properties of the tissues.
Doppler ultrasonography
Ultrasound signals reflected off stationary surfaces retain thesame frequency with which they were transmitted, but the principle underlying Doppler ultrasonography is that the frequency of signals reflected from moving objects such as red blood cells shifts in proportion to the velocity of the target. The output from a continuous wave Doppler ultrasonograph is usually presented as an audible signal, so that a sound is heard whenever there is movement of blood in the vessel being examined.
Pulsed ultrasonography
Continuous wave ultrasonography provides little scope for restricting the area of tissue that is being examined because any sound waves that are intercepted by the receiving crystal will produce an output signal. The solution is to use pulsed ultrasonography. The investigator can focus on a specific tissue plane by transmitting a pulse of ultrasound and closing the receiver except when signals from a predetermined depth are returning. This allows, for example, the centre of an artery and the areas close to the vessel wall to be examined in turn.
Duplex scanners
An important advance in vascular ultrasonography has been the development of spectral analysis, which delineates the complete spectrum of frequencies (that is, blood flow velocities) found in the arterial waveform during a single cardiac cycle. The normal (“triphasic”) Doppler velocity waveform is made up of three components which correspond to different phases of arterial flow: rapid antegrade flow reaching a peak during systole, transient reversal of flow during early diastole, and slow antegrade flow during late diastole. Doppler examination of an artery distal to a stenosis will show characteristic changes in the velocity profile: the rate of rise is delayed, the amplitude decreased, and the transient flow reversal in early diastole is lost. In severe disease, the Doppler waveform flattens; in critical limb ischaemia it may be undetectable. Examination of an arterial stenosis shows an increase in blood velocity through the area of narrowing. The site(s) of any stenotic lesions can be identified by serial placement of the Doppler probe along the extremities. The criteria used to define a stenosis vary between laboratories, but a twofold increase in peak systolic velocity compared with the velocity in an adjacent segment
By combining the pulsed Doppler system with real time B mode ultrasound imaging of vessels, it is possible to examine Doppler flow patterns in a precisely defined area within the vessel lumen. This combination of real time B mode sound imaging with pulsed Doppler ultrasonography is called duplex scanning. The addition of colour frequency mapping (so called colour duplex or triplex scanners) makes the identification of arterial stenoses even easier and reduces the scanning tim
Friday, October 8, 2010
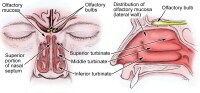
Anatomy of Olfactory System
Introduction The olfactory system represents one of the oldest sensory modalities in the phylogenetic history of mammals. Olfaction is less developed in humans than in other mammals such as rodents. As a chemical sensor, the olfactory system detects food and influences social and sexual behavior. The specialized olfactory epithelial cells characterize the only group of neurons capable of regeneration. Activation occurs when odiferous molecules come in contact with specialized processes known as the olfactory vesicles. Within the nasal cavity, the turbinates or nasal conchae serve to direct the inspired air toward the olfactory epithelium in the upper posterior region. This area (only a few centimeters wide) contains more than 100 million olfactory receptor cells. These specialized epithelial cells give rise to the olfactory vesicles containing kinocilia, which serve as sites of stimulus transduction.
The image below depicts olfactory anatomy.
Olfactory Epithelium
The olfactory epithelium consists of 3 cell types, basal, supporting, and olfactory receptor cells. Basal cells are stem cells that give rise to the olfactory receptor cells. The continuous turnover and new supply of these neurons are unique to the olfactory system. In no other location in the mature nervous system do less differentiated stem cells replace neurons. Supporting cells are scattered among the receptor cells and have numerous microvilli and secretory granules, which empty their contents onto the mucosal surface. The receptor cells are actually bipolar neurons, each possessing a thin dendritic rod that contains specialized cilia extending from the olfactory vesicle and a long central process that forms the fila olfactoria. The cilia provide the transduction surface for odorous stimuli.
The vomeronasal organ is a specialized bilateral membranous structure located in the base of the anterior nasal septum, at the junction of the septal cartilage and the bony septum. It is believed to detect external chemical signals called pheromones. These signals, which are not detected consciously as odors by the olfactory system, mediate human autonomic, psychological, and endocrine responses.
The trigeminal nerve innervates the posterior nasal cavity to detect noxious stimuli.
Olfactory Nerve and the Cribriform Plate
The small unmyelinated axons of the olfactory receptor cells form the fine fibers of the first cranial nerve and travel centrally toward the ipsilateral olfactory bulb to make contact with the second-order neurons. Conduction velocities are extremely slow, and support is provided in bundles by a single Schwann cell. As previously mentioned, the trigeminal nerve (cranial nerve V) sends fibers to the olfactory epithelium to detect caustic chemicals such as ammonia. The cribriform plate of the ethmoid bone, separated at the midline by the crista galli, contains multiple small foramina through which the olfactory nerve fibers, or fila olfactoria, traverse. Fracture of the cribriform plate in traumatic settings can disrupt these fine fibers and lead to olfactory dysfunction.
Olfactory Bulb
The olfactory bulb lies inferior to the basal frontal lobe. The olfactory bulb is a highly organized structure composed of several distinct layers and synaptic specializations. The layers (from outside toward the center of the bulb) are differentiated as follows:
- Glomerular layer
- External plexiform layer
- Mitral cell layer
- Internal plexiform layer
- Granule cell layer
Mitral cells are second-order neurons contacted by the olfactory nerve fibers at the glomerular layer of the bulb. The glomerular layer is the most superficial layer, consisting of mitral cell dendritic arborizations (glomeruli), olfactory nerve fibers, and periglomerular cells. Periglomerular cells contact multiple mitral cell dendrites within the glomeruli and provide lateral inhibition of neighboring glomeruli while allowing excitation of a specific mitral cell dendritic tree. Each mitral cell is contacted by at least 1000 olfactory nerve fibers.
The external plexiform layer contains the passing dendrites of mitral cells and a few tufted cells, which are similar in size to mitral cells. Some of the granule cell dendrites in the plexiform layer contact mitral cell dendrites through a specialized dendrodendritic synapse, which also is termed a reciprocal synapse (vesicles seen within both presynaptic and postsynaptic membranes).
Tufted cells also receive granule cell input through dendrodendritic and dendrosomatic contact. Pyramidal mitral cells are the largest neurons in the bulb and are located in a narrow band between the external and internal plexiform layers. The granule cell layer contains multiple small round neurons that lack axons. Long dendritic processes of the neurons reach the more superficial layers and inhibit mitral cells and tufted cells. Small distal processes make contacts with the exiting mitral cell axons.
Olfactory Tract and Central Pathways
Mitral cell axons project to the olfactory cortex via the olfactory tract. Medial fibers of the tract contact the anterior olfactory nucleus and the septal area. Some fibers project to the contralateral olfactory bulb via the anterior commissure. Lateral fibers contact third-order neurons in the primary olfactory cortex (prepyriform and entorhinal areas) directly. Third-order neurons send projections to the dorsomedial nucleus of the thalamus, the basal forebrain, and the limbic system.
The thalamic connections are thought to serve as a conscious mechanism for odor perception, while the amygdala and the entorhinal area are limbic system components and may be involved in the affective components of olfaction. Investigations of regional cerebral blood flow have demonstrated a significant increase in the amygdaloid nucleus with the introduction of a highly aversive odorant stimulus, and this has been associated with subjective perceived aversiveness.
Central Projections
The pyriform lobe includes the olfactory tract, the uncus, and the anterior part of the parahippocampal gyrus. The prepyriform and the periamygdaloid areas of the temporal lobe represent the primary olfactory cortex. The entorhinal area is known as the secondary olfactory cortex and is included in the pyriform lobe. The olfactory system is the only sensory system that has direct cortical projections without a thalamic relay nucleus. The dorsomedial nucleus of the thalamus receives some olfactory fibers that ultimately reach the orbitofrontal cortex.
The anterior olfactory nucleus receives collateral fibers from the olfactory tract and projects to the contralateral olfactory bulb and anterior olfactory nucleus via the anterior commissure.
The region of anterior perforated substance contains cells that receive direct mitral cell collaterals and input from the anterior olfactory nucleus, amygdaloid nucleus, and temporal cortex. This area ultimately projects to the stria medullaris and the medial forebrain bundle.
Using the uncinate fasciculus, the entorhinal area sends projections to the hippocampal formation, anterior insula, and frontal cortex.
Clinical Correlation
As many as 2 million people in the United States experience some type of olfactory dysfunction, causes of which include head trauma, upper respiratory infections, tumors of the anterior cranial fossa, and exposure to toxic chemicals or infections. The following terms are used to describe the degree of smell aberration:
- Anosmia - Absence of smell sensation
- Hyposmia - Decreased sensation
- Dysosmia - Distortion of smell sensation
- Cacosmia - Sensation of a bad or foul smell
- Parosmia - Sensation of smell in the absence of appropriate stimulus
Olfactory dysfunction is a hallmark of certain syndromes such as Kallmann syndrome (ie, hypogonadism with anosmia) and Foster Kennedy syndrome (ie, papilledema, unilateral anosmia, and optic atrophy usually associated with an olfactory groove meningioma).
The classic description of partial complex epilepsy with a mesial temporal focus includes an aura of foul-smelling odors (termed uncinate fits) that occur before seizure onset, emphasizing presumed origination at the uncus.
Olfactory dysfunction is associated with early Parkinson disease and with other neurodegenerative disorders such as Alzheimer disease and Huntington chorea.1 An association also exists between abnormal olfactory identification and obsessive-compulsive disorder.2
Head trauma leading to fracture of the cribriform plate may cause cerebrospinal fluid (CSF) rhinorrhea and a potential for meningitis. Paranasal sinus endoscopy may lead to violation of the cribriform plate and potential infectious complications. Olfactory structures also can be injured during craniotomies involving the anterior cranial base or from subarachnoid hemorrhage, which may disrupt the fine fibers of the olfactory nerve.
Clinical Evaluation
Detection of olfactory dysfunction begins with sampling of a series of common odors, which can be performed at the bedside with odiferous substances such as coffee, lemon, and peppermint. Tests, including those developed at the Connecticut Chemosensory Clinical Research Center (CCCRC), have aided examiners in identification of abnormalities in odor detection and discrimination. The University of Pennsylvania Smell Identification Test (UPSIT) is another useful tool; it consists of 40 items for evaluation of olfactory and trigeminal nerve function in the nasal cavity.
Central hyposmia may manifest as abnormalities in odor recognition rather than odor detection. Thorough evaluation of patients who have anosmia includes imaging of anterior cranial structures. The clinician should always counsel patients with anosmia regarding sensory loss, including potential risks associated with the lack of smell sensation (eg, inability to detect dangers such as smoke, spoiled foods, toxins).
Promptly complete evaluation and treatment of clear rhinorrhea in the patient in whom leakage of CSF is suspected. Initial testing of fluid for glucose suggests CSF but is not confirmatory. Presence of beta-transferrin is a more sensitive indicator of CSF rhinorrhea. Computed tomography with cisternography or radionuclide scans can be used to detect the site of CSF leakage from the anterior cranial fossa. Repair of leaks at the level of the cribriform plate may be achieved from the intracranial approach, intranasal (endoscopic) approach, or both, depending on the nature of the defect.
Positron emission tomography (PET) and functional MRI are promising modalities to assist in making the diagnosis of different types of hyposmia (central vs peripheral), as well as in delineation of the role of limbic structures as sites of odor recognition, memory, and integration of multisensory inputs.
Wednesday, October 6, 2010
Anatomy in Cutaneous Surgery
Skin Tension Lines
Skin tension lines (STLs) are the result of a complex interaction between internal and external factors involving the skin. The intrinsic framework, which consists of elastin and collagen, progressively loosens with age. Their interaction with the muscles of facial expression leads to the development of STLs. Generally, STLs are perpendicular to the underlying muscles of the face. Aging, particularly photoaging, tends to accentuate the appearance of STLs.
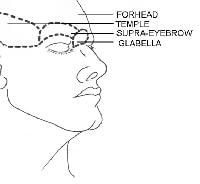
In the repair of STLs, the correct placement of the long axis of an excision parallel to the STLs results in better scar cosmesis. Furthermore, flaps should be placed to allow the suture lines to fall in STLs. Although STLs may vary between individuals, some areas of the face have greater variability than others. Typically, the forehead, which has 1 major muscle group that pulls it vertically, has little individual variability; nearly everyone has horizontal STLs. In comparison, anatomic areas where multiple muscles act in different directions are likely to have greater variability.
In elderly patients, the direction of the relaxed STLs is generally obvious. In areas of ambiguity, excising the lesion as a circle and undermining it invariably pulls the surgical defect into an oval with the long axis corresponding to the relaxed STL.Cosmetic Units and Subunits
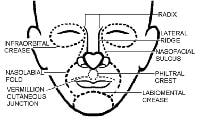
Junction lines are fixed landmarks that separate the cosmetic units of the face.
Placing the suture lines on these boundaries (eg, eyebrow, nasolabial fold) optimizes scar formation. When a surgical wound is closed, repairing the wound in a cosmetic unit along a junction line is best. In larger defects that require a flap, the best results are achieved by using tissue from the same or adjacent cosmetic unit and by placing suture lines on the boundaries of those units. Subunits within cosmetic units are often subtle and individually variable. Paying attention to subtleties such as color, texture, sebaceous features, and hair characteristics help in identifying the changes between the subunits.The scalp and forehead are individual cosmetic units that are separated by the hairline. In a bald individual, the top horizontal forehead crease serves as the junction line. Subunits of the forehead include the glabella, temples, and eyebrows.
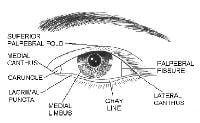
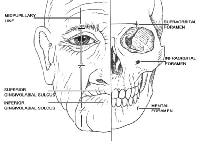
The eyelids are a complex structure with multiple subunits that mimic the underlying orbicularis oculi muscle. The largest component of the eyelid is the orbital portion, which borders the eyebrow superiorly and the cheek inferiorly. Just below the eyebrow is the preseptal area and then the pretarsal portion where eyelashes insert. Additional components of the eyelid include the superior palpebral fold, the palpebral fissure, the medial limbus, and the medial canthus.
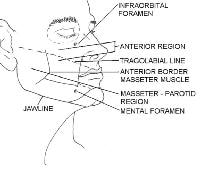
The cheek region is subdivided by the anterior prominence of the clenched masseter muscle. The masseter-parotid region lies posterior to this landmark and is posteriorly bound by the ear. The mandibular region lies anterior to the masseter and inferior to the lower lip. The malar subunit is around the zygoma anterior to the masseter muscle. This subunit is referred to as the anterior region.
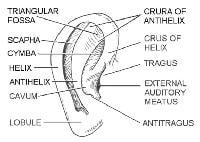
The subdivisions of the external ear allow for good clinical descriptions of skin lesions in this location.
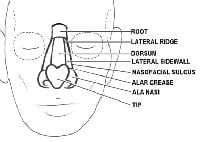
The nose1 has the most subdivisions on the face.
The horizontal root, which borders the glabella on the forehead, is positioned superiorly. The mid nose contains the dorsum medially and is flanked by the 2 lateral sidewalls. The dorsum is inferiorly bordered by the tip, which ends in the columella. The columella is the thin sliver of tissue that separates the nostrils on the underside of the nose. The tip is bordered by the ala nasi, or alae, on both sides, and the columella is flanked by the soft triangles, which also border the tip and the alae.
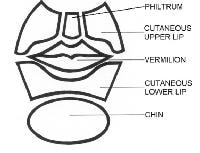
The lower part of the face is dominated by the subunits of the lip.
Below the nose in the moustache area are the cutaneous regions of the upper lip, which are separated from the cheek by the nasolabial fold. The middle depression below the nose, known as the philtrum, is an important anatomic subunit because even minimal displacement of this structure results in significant disfigurement. The lips constitute the vermilion subunit. The cutaneous lower lip, which borders the chin inferiorly and is bound by the nasolabial fold laterally, is below the vermillion.
Muscles of Facial Expression and the Superficial Musculoaponeurotic System
Muscles of facial expression
The muscles of facial expression are unique in a number of ways. Rather than inserting into bones or tendons, all of the muscles of facial expression originate from or insert into the skin. They are all derived from the second embryonic branchial arch and are innervated by the seventh cranial (facial) nerve. Different anatomic areas of the face have synergistic and antagonist groups of muscles that enable individuals to make varied facial expressions.
Muscles affecting the forehead and eyebrow include the frontalis muscle, which creates the horizontal wrinkles on the forehead and assists with eyebrow elevation, and the corrugators and procerus muscles, which are antagonistic muscles on the forehead. The orbicularis oculi muscles are a complex of muscles surrounding the eyes; these assist with closing the eye tightly. This muscle lies superficially in the eyelid skin and is encountered with even a shallow incision. The dominant muscle of the nose is the nasalis muscle, which consists of nasal and alar components. Its function is to compress and dilate the nares.
The mouth has the most extensive network of facial musculature and accounts for much of an individual's capability of facial expression. The orbicularis oris encircles the mouth and is the major component of the lips. The major functions of the orbicularis oris muscle are to pull the lips against the teeth, to draw the lips together, to pull the corners of the mouth together, and to pucker. This muscle is also extremely important for the phonation of sounds that rely on the lips, such as the pronunciation of the letters M, V, F, and P.
A group of 6 muscles, collectively known as the quadratus labii superioris muscle, controls the upper mouth. The 6 muscles are as follows:
- The zygomaticus major muscle starts from the posterolateral zygomatic bone and travels medially to insert on the upper portion of the orbicularis oris muscle. The zygomaticus major muscle helps in forming the lower nasolabial fold and is primarily responsible in smiling.
- The zygomaticus minor muscle arises just medially to the zygomaticus major and assists with its functions.
- The levator labii superioris muscle arises from the inferior portion of the maxilla and inserts on the upper lip, more medially than the zygomaticus muscles. The levator labii superioris muscle helps elevate the medial part of the upper lip and assists the zygomatic muscles with open smiling. The levator and zygomaticus muscles form the nasolabial fold.
- The levator anguli oris muscle is the most deeply positioned of the lip elevators and inserts on the upper corner of the mouth to assist with lip elevation.
- The risorius muscle arises over the parotid gland, inserts into the skin and mucosa of the lateral corner of the mouth, and assists with smiling. The risorius is not always present.
- The buccinator muscle is neither an elevator nor a depressor of the lip. It arises just posterior and medial to the last molar tooth and extends forward to become continuous with the orbicularis oris muscle. The buccinator muscle is the major component of the cheek musculature and prevents overdistension of the cheek (eg, in playing a wind instrument). This muscle assists the orbicularis oris muscle in whistling.
The depressors of the lip include the depressor anguli oris, the depressor labii inferioris, and the mentalis muscles. The marginal mandibular branch of the facial nerve innervates the depressors of the lip.
- The depressor anguli oris muscles arise from the lateral part of the mandible and travel superomedially to insert, with the orbicularis oris muscle, in the corners of the mouth. They function to depress and retract the corners of the mouth.
- The depressor labii inferioris muscles arise more medially on the mandible and travel superiorly to insert, with the orbicularis oris muscle, in the lower and medial part of the lip. Similar to the depressor anguli oris muscle, these muscles assist with the depression and retraction of the lower lip.
- The mentalis muscle, which is deep to both the depressor anguli oris and labii inferioris muscles, arises from the mandible and lower lateral incisor and courses inferiorly to insert on the skin covering the chin. The mentalis muscle elevates and wrinkles the chin and assists in protruding the lower lip.
Superficial musculoaponeurotic system
The facial musculature must work synergistically to allow for a wide range of facial expressions. The superficial musculoaponeurotic system (SMAS) is a discrete fibromuscular layer that envelops and interlinks the muscles to provide these synergies. The SMAS delineates the dissection planes for the extensive undermining necessary in facial rejuvenation procedures.
In addition, the SMAS serves as a useful marker in assessing the location of vital blood vessels and nerves. The superficial portion of the SMAS generally houses the axial blood vessels and sensory nerves, whereas the deeper levels contain the more vital motor nerves. The SMAS is generally located beneath the subcutaneous fat and superficial to the muscles. Superior to the zygoma, the SMAS links the temporalis, frontalis, occipitalis, and procerus muscles into a freely moveable continuous plane that connects with the subgaleal space. Inferiorly, the fascial anatomy is unclear; however, the SMAS interconnects the platysma, risorius, and depressor anguli oris muscles inferior to the zygoma. The muscles of the medial aspect of the face, including the orbicularis oculi, lip elevator, and nasal muscles, are not ensheathed by an interconnected SMAS.
Nerves
Sensory nervesThe trigeminal nerve, or cranial nerve (CN) V is primarily responsible for the sensory innervation of the face. The cervical, facial, glossopharyngeal, and vagus nerves have smaller contributions. The sensory nerves are typically located more superficially than the motor nerves, along the junction of the fat and the SMAS. Transection of the sensory nerves does not result in the serious morbidity that motor nerve damage causes, and the recovery of sensory function after such injury is typical.
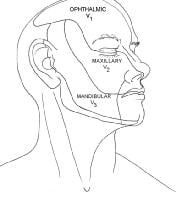
The trigeminal nerve is divided into 3 branches: ophthalmic (CN V1), maxillary (CN V2), and mandibular (CN V3).The V1 division provides sensation to the anterior part of the scalp, forehead, upper eyelid, and nasal bridge. Branches that arise around the superior orbital rim include the supraorbital, supratrochlear, infratrochlear, external nasal, and lacrimal branches. The V2 division supplies sensation to the lower eyelid, nasal sidewalls and columella, temple, and upper lip. Its major branch is the infraorbital nerve, which emerges from the infraorbital foramen with the infraorbital artery and vein. Other smaller branches include the zygomaticofacial and zygomaticotemporal nerves. The V3 division is the largest and most complicated of the divisions of CN V. It is the only division that carries motor fibers. The mandibular nerves provide sensation to the lower lip, chin, mandible, and preauricular areas.
The auriculotemporal, buccal, and mental nerves are the 3 major cutaneous branches of the mandibular nerve. The auriculotemporal nerve sends sensory fibers to the auricles, temples, and temporal parietal aspect of the scalp. In addition, it provides sensation to the external auditory canals, eardrums, and temporomandibular joints, and it carries some secretory fibers to the parotid glands. The buccal nerve is inaccessible for nerve blocks because of its deep location. It sends fibers to the cheek, mucosa, and gingiva. The mental nerve is the continuance of the inferior alveolar nerve, and it emerges from the mental foramen on the chin. It provides sensation to the chin, lower lip, mucosa, and gingiva of the lower lip. The motor component of the trigeminal nerve primarily innervates the muscles of mastication.
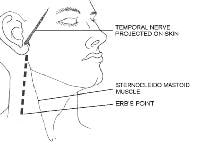
The cervical plexus lies deep to the sternocleidomastoid muscle. The plexus provides sensation to several important structures and is derived from C2 through C4. These nerves include the great auricular (C2, C3), lesser occipital (C2), greater occipital (C2), third occipital (C3), transverse cervical (C2, C3), and supraclavicular nerves. They send sensory fibers to the neck, posterior part of the ear, and postauricular scalp. The spinal accessory and cervical nerves emerge near the Erb point in the posterior triangle on the neck and are easily damaged during cutaneous surgery.
Lastly, sensory branches of the vagus, glossopharyngeal, and facial nerves innervate the skin of the external auditory canal, the concha, and the posterior sulcus. Awareness of the sensory branches of the face allows the use of nerve blocks, which provide effective anesthesia with minimal discomfort for the patient. Mental, infraorbital, and supraorbital blocks are easily achieved after the identification of their respective foramina, which lie in the midpupillary plane.Foramina for nerve blocks. Illustrated by Charles Norman.
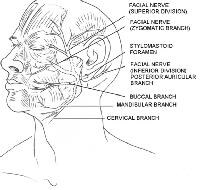
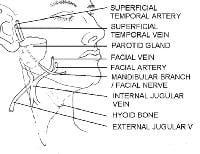
CN VII, also known as the facial nerve, provides motor innervation to all the muscles of facial expression. CN VII also provides motor fibers to the digastric, stylohyoid, and stapedius muscles. In addition, sensory innervation to the anterior two thirds of the tongue, external auditory meatus, soft palate, and pharynx is mediated via the facial nerve. The motor portion of the facial nerve is divided into 5 major branches, but individual variation is common, with numerous smaller arborizations emanating from each major branch. The main facial nerve trunk emerges from the stylomastoid foramen, which is covered by the mastoid process, and along the posterior deep portion of the parotid gland. The main facial nerve trunk then divides into the temporal, zygomatic, buccal, mandibular, and cervical branches.
Branches of the facial nerve. Illustrated by Charles Norman.
The temporal branch innervates the muscles of the upper part of the face including the upper orbicularis oculi, frontalis, and corrugator muscles. This branch is extremely susceptible to inadvertent injury because it travels superficially when it crosses the middle portion of the zygoma.
Course of the temporal nerve and location of the Erb point. Illustrated by Charles Norman.
Transection of the temporal branch most prominently leads to unilateral frontalis dysfunction, which leaves the patient with ptosis and the inability to raise his or her eyebrows.
The zygomatic branch provides motor fibers to the lower orbicularis oculi, procerus, some lip elevator, and some nasal muscles.
The zygomatic branch provides motor fibers to the lower orbicularis oculi, procerus, some lip elevator, and some nasal muscles.
The buccal branch often has numerous anastomotic connections with the zygomatic branch and sends fibers to similar muscles, in addition to the buccinator, orbicularis oris, depressor anguli oris, and risorius muscles. The buccal and zygomatic branches travel superficially over the buccal fat pad and just below the SMAS. This orientation makes them susceptible to injury during face-lift procedures. Transection of the nerves of the zygomatic and buccal branch leads to unpredictable defects, because muscular innervation in the mid face is variable.
In general, the marginal mandibular nerve does not have anastomotic connections. It innervates the orbicularis oris and lip depressor muscles. The anatomic course of the marginal mandibular nerve is unpredictable, but it should be considered in any excision near the angle of the mandible and the inferior margin of the parotid gland. Transection of the marginal mandibular nerve leads to extreme cosmetic and functional loss because the muscles of the mouth enable a significant amount of facial expression. The marginal mandibular branch of the facial nerve has a superficial course near the mandible and chin. This nerve usually lies anterior to the facial artery, which is palpable anterior to a clenched masseter muscle.Course of the marginal mandibular nerve. Illustrated by Charles Norman.
Transection of this nerve results in a droopy lip and subsequently drooling. Excisions on the lips can also lead to drooling, but not because of the transection of a motor nerve in this location.
The cervical branch is posterior and deep to the marginal mandibular nerve and innervates the platysma muscle. The cervical branch is of little importance to the cutaneous surgeon because its transection does not result in great functional or cosmetic loss.
The cutaneous surgeon must be aware of the delayed effects of local anesthetic on motor fibers. The unmyelinated sensory fibers lose conduction instantaneously, whereas the deeper myelinated motor fibers may lose their function only after a prolonged procedure with possibly greater anesthetic volume. Thus, the surgeon should not be alarmed by the delayed onset of a facial muscle paralysis. This effect on the motor nerves can last as long as 12 hours, and the patient should be appropriately counseled.
Blood Vessels
The external carotid artery provides most of the arterial blood supply to the face; the internal carotid artery makes a smaller contribution. The external carotid artery is the origin of the facial artery deep to the mandible near the pharynx. The facial artery courses around the jaw anterior to the masseter, where it is palpable, and continues superomedially to end as the angular artery near the medial canthus. Along its path, the facial artery serves as the origin to the inferior and superior labial arteries as well as smaller nasal branches. It provides the arterial blood supply to the lips and the middle of the face. At its terminal point, the facial artery connects with the ophthalmic artery, which provides important anastomoses with the internal carotid system.
Prior to its terminal differentiation, the external carotid artery results in the occipital and posterior auricular arteries, which supply the posterior part of the scalp and the postauricular areas. The terminal branches of the external carotid artery are the superficial temporal and internal maxillary arteries.
The superficial temporal artery arises in the parotid gland superficial to the branches of the facial nerve. The artery courses superiorly and results in the horizontal transverse facial artery 2 cm inferior to the zygoma; the resultant artery connects with the facial artery. As it continues superiorly, the superficial temporal artery also becomes more superficial and is palpable posterior to the temporal mandibular joint and anterior to the ear. The superficial temporal artery terminates in the parietal and frontal branches, which deliver blood to the scalp. Along its course, the superficial temporal artery provides blood to the lateral part of the face, the temple, the forehead, and the scalp. It also serves as the origin for smaller middle temporal and zygomatico-orbital arteries that supply some midfacial structures.
The internal maxillary is a deep artery that forms where the superficial temporal artery arises from the external carotid artery. Because of its deep location, the cutaneous surgeon rarely encounters the internal maxillary artery. Important branches of this artery include the infraorbital artery and the inferior alveolar artery, which continues through the mental foramen as the mental artery to provide blood to the chin.
The internal carotid artery supplies arterial blood to the eyelids, the upper and dorsal parts of the nose, the lower part of the forehead, and the scalp. The important major branch of the internal carotid artery is the ophthalmic artery. The ophthalmic artery has supraorbital, supratrochlear, infratrochlear, dorsal nasal, and external nasal branches that may form anastomoses with the external carotid system through the angular artery.
The venous network of the face parallels the arterial system. Unlike the arteries, the veins tend to be straighter and less tortuous. The facial vein parallels the facial artery and drains blood from the middle of the face into the internal jugular vein. The venous system of the medial aspect of the face (the dangerous triangle) involves the drainage of the upper lip and paranasal areas. In this area, the facial vein directly connects with the cavernous sinus, via the ophthalmic vein or indirectly connects with it, via the pterygoid plexus. The lateral part of the face and the scalp drain into the superficial temporal and retromandibular veins, which lead into the external jugular vein.
Lymphatics
The cutaneous surgeon often removes malignancies that can cause lymph node metastases. Knowledge of lymph flow is essential for an adequate clinical examination of the lymph nodes. Lymph flows from superficial areas to deep areas and then flows along 4 major tracts in an inferolateral direction to the collecting nodes in the neck and jaw. Great individual variability exists in the lymph flow; however, a general framework exists.
All the lymphatic fluid from the face eventually collects in a triangle of lymph nodes in the neck. The transverse cervical chain forms the inferior horizontal leg of the triangle, the spinal accessory chain forms the lateral leg, and the internal jugular chain forms the medial leg. The superior point of the triangle includes the superficial cervical and parotid nodes. The legs of the triangle are known as the deep lateral cervical nodes.
At the junction of the internal jugular and transverse cervical chains, the lymphatics enter the venous circulation at the jugulosubclavian junction. The spinal accessory chain travels along the spinal accessory nerve, making its removal more treacherous. This chain may be involved with early metastases from malignancies in the nasopharyngeal and thyroid areas. The transverse cervical chain is found along the transverse cervical vessels above the clavicle. The internal jugular chain is the major collection point for the head and neck, and it is divided into anterior and lateral divisions. By turning the patient's chin ipsilaterally and by rolling the relaxed sternocleidomastoid muscles between his or her fingers, the physician can clinically examine this group.
The postauricular node can be single or multiple, and it is located in the mastoid area attached to the insertion of the sternocleidomastoid muscle. Drainage from the ear can course anteriorly to the parotid nodes or inferiorly to the spinal accessory and internal jugular chains. Superficial and deep occipital nodes drain the posterior aspect of the scalp and the nuchal area. These nodes subsequently flow into the spinal accessory chain.
The parotid nodes have extraglandular and intraglandular components. The extraglandular nodes include the preauricular and infra-auricular subnodes and are located in the parotid fascia. These nodes function as a unit with the intraglandular nodes, which are deep in the gland. The parotid nodes drain into the submandibular chain or transverse cervical chain.
The facial nodes drain the mid face and are extremely variable between individuals. From superior to inferior, they are the infraorbital, malar, buccinator, and mandibular nodes. When present, these nodes are found in the subcutaneous layer above the muscles of the face. The facial nodes drain into the submandibular and submental chains.
The submandibular nodes are divided into 5 groups on the basis of their relationship to the facial vein and the submandibular gland. The preglandular nodes are below the platysma and anterior to the gland. The prevascular node is in the precarious position on the facial artery and touches the marginal mandibular nerve.
The postvascular nodes are also adjacent to the motor nerve. The existence of a retroglandular group is disputed. The submandibular gland surrounds a large group of intracapsular nodes. Many individuals have palpable nodes in the submandibular triangle. For clinical examination, the submandibular nodes can be palpated when the patient relaxes his or her neck muscles and moves his or her chin downward.
The submental nodes are above the mylohyoid and deep to the platysma in the submental triangle. The nodes drain the middle two thirds of the lower lip, the medial aspect of the cheek, and some facial nodes, and they can drain into the ipsilateral and contralateral nodes in the neck. Examination of the submental nodes is best accomplished by bimanually palpating the floor of the mouth and by pushing up under the patient's chin.
The superficial lateral cervical nodes receive drainage from the parotid, submandibular, and postauricular nodes. These cervical nodes are located on the superior external jugular vein, and some believe that they are part of the parotid infra-auricular nodes. The superficial lateral cervical nodes drain into the internal jugular chain.
The key cutaneous muscles of the neck are the platysma and sternocleidomastoid muscles. The platysma is covered by the SMAS, which is continuous with the lower muscles of the face, and it is also considered a muscle of facial expression. The platysma is an extremely thin muscle that is superficial in the neck. The sternocleidomastoid muscle extends from the medial clavicle to the postauricular area and divides the neck into posterior and anterior triangles.
Key components of the anterior triangle include the internal and external carotid arteries, the internal jugular vein, and the vagus and hypoglossal nerves. The important structure to consider to the posterior triangle is the spinal accessory nerve (CN XI), which innervates the sternocleidomastoid and trapezius muscles. Transection of the spinal accessory nerve results in a winged scapula and difficulty with arm abduction. The superficial cervical plexus is also found in the posterior triangle. The superficial cervical plexus has sensory, motor, and sympathetic functions.
A crucial anatomic landmark in the posterior triangle is the Erb point. The spinal accessory, great auricular, lesser occipital, and transverse cervical nerves all pass within 2 cm above or below this location. The Erb point can be located by drawing a line between the angle of the mandible and the mastoid process with the patient's head slightly turned. The Erb point lies at the junction of the posterior border of the sternocleidomastoid muscle and the point 6 cm inferior to the midpoint of the line drawn.
Blood vessels of the neck
The key blood vessels of the neck include the common and external carotid arteries and the jugular veins. The common carotid, internal jugular, and vagus nerves are found in the carotid sheath under the sternocleidomastoid and infrahyoid muscles before the carotid arteries bifurcate into their external and internal branches.
The external jugular vein forms below the parotid gland and travels inferiorly along the surface of the sternocleidomastoid muscle. The external jugular vein empties into the subclavian or internal jugular veins after it pierces through the superficial portion of the deep cervical fascia in the posterior triangle.
Skin tension lines
STLs typically lie in a transverse direction on the neck. The placement of excision lines in this orientation is essential because hypertrophic or reddened scars often result from misplaced excisions on the neck.
When designing flaps, borrow tissue from the same or adjacent cosmetic units to minimize anatomic distortion and maximize tissue match.
A reservoir of skin for flaps or primary closure can be found on the lower and posterior part of the cheek near the angle of the mandible. In older persons, this area is the jowl. Other areas with redundant skin include the preauricular aspect of the cheek, the temple, and the neck.
Subunits of the anterior region. Illustrated by Charles Norman.Cutaneous Anatomy of the Neck
Muscles of the neckThe key cutaneous muscles of the neck are the platysma and sternocleidomastoid muscles. The platysma is covered by the SMAS, which is continuous with the lower muscles of the face, and it is also considered a muscle of facial expression. The platysma is an extremely thin muscle that is superficial in the neck. The sternocleidomastoid muscle extends from the medial clavicle to the postauricular area and divides the neck into posterior and anterior triangles.
Key components of the anterior triangle include the internal and external carotid arteries, the internal jugular vein, and the vagus and hypoglossal nerves. The important structure to consider to the posterior triangle is the spinal accessory nerve (CN XI), which innervates the sternocleidomastoid and trapezius muscles. Transection of the spinal accessory nerve results in a winged scapula and difficulty with arm abduction. The superficial cervical plexus is also found in the posterior triangle. The superficial cervical plexus has sensory, motor, and sympathetic functions.
A crucial anatomic landmark in the posterior triangle is the Erb point. The spinal accessory, great auricular, lesser occipital, and transverse cervical nerves all pass within 2 cm above or below this location. The Erb point can be located by drawing a line between the angle of the mandible and the mastoid process with the patient's head slightly turned. The Erb point lies at the junction of the posterior border of the sternocleidomastoid muscle and the point 6 cm inferior to the midpoint of the line drawn.
The key blood vessels of the neck include the common and external carotid arteries and the jugular veins. The common carotid, internal jugular, and vagus nerves are found in the carotid sheath under the sternocleidomastoid and infrahyoid muscles before the carotid arteries bifurcate into their external and internal branches.
The external jugular vein forms below the parotid gland and travels inferiorly along the surface of the sternocleidomastoid muscle. The external jugular vein empties into the subclavian or internal jugular veins after it pierces through the superficial portion of the deep cervical fascia in the posterior triangle.
Skin tension lines
STLs typically lie in a transverse direction on the neck. The placement of excision lines in this orientation is essential because hypertrophic or reddened scars often result from misplaced excisions on the neck.
Anatomic Surgical Pearls
Free margins are located on the eyelids, helices of the ears, lips, and alar rim and columella. Unopposed tension caused by a surgical repair may distort these structures. Wound closure in these locations often require flap and/or graft placement to lessen the risk.When designing flaps, borrow tissue from the same or adjacent cosmetic units to minimize anatomic distortion and maximize tissue match.
A reservoir of skin for flaps or primary closure can be found on the lower and posterior part of the cheek near the angle of the mandible. In older persons, this area is the jowl. Other areas with redundant skin include the preauricular aspect of the cheek, the temple, and the neck.
Place the suture lines along STLs and on the boundaries of the cosmetic units whenever possible.
Preoperatively identify the vital structures that might be damaged in the operative field, and stay vigilant to avoid them. Examples of vital structures include the temporal nerve in the upper part of the cheek and temple, the marginal mandibular nerve on the jaw line, and CN XI at the Erb point.
Identify sensory innervation to structures in the operative field, and perform a nerve block wherever possible to minimize the patient's discomfort and distortion of the operative field by using large amounts of lidocaine. Examples of sensory innervations include the mental nerve on the chin and the supraorbital and supratrochlear nerves in large lesions on the forehead.
When working in deeper planes, attempt to identify the SMAS, which can help in locating and avoiding vital vessels and nerves.
Preoperatively identify the vital structures that might be damaged in the operative field, and stay vigilant to avoid them. Examples of vital structures include the temporal nerve in the upper part of the cheek and temple, the marginal mandibular nerve on the jaw line, and CN XI at the Erb point.
Identify sensory innervation to structures in the operative field, and perform a nerve block wherever possible to minimize the patient's discomfort and distortion of the operative field by using large amounts of lidocaine. Examples of sensory innervations include the mental nerve on the chin and the supraorbital and supratrochlear nerves in large lesions on the forehead.
When working in deeper planes, attempt to identify the SMAS, which can help in locating and avoiding vital vessels and nerves.
Subscribe to:
Posts (Atom)